Solving Recruitment Challenges
with Proven Results
Delays in participant recruitment and retention are among the top causes of clinical trial slowdowns, leading to budget overruns and lost time in bringing therapies to market. At Linear, we solve this challenge head-on with an innovative, proven approach that streamlines volunteer recruitment and retention.
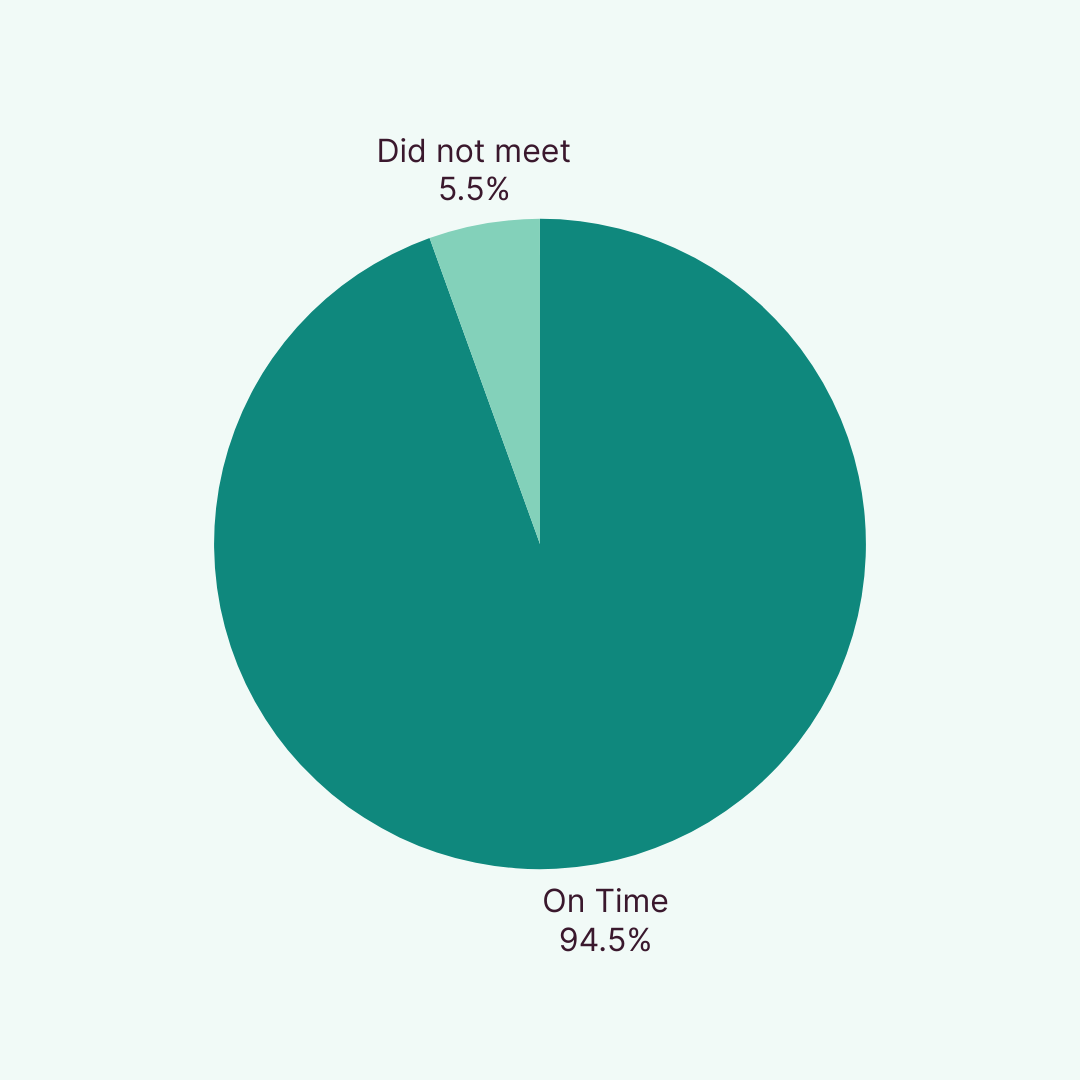
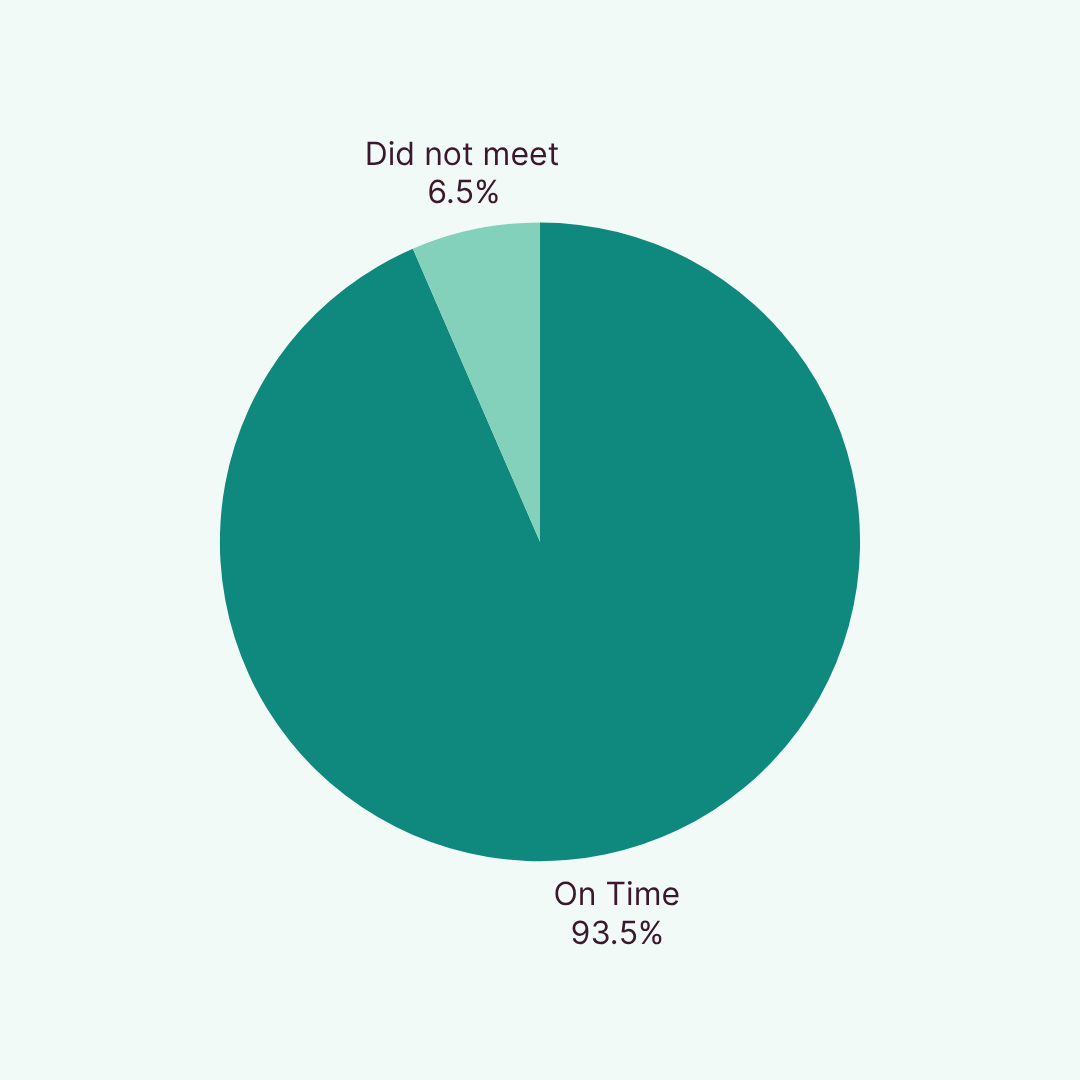
We successfully randomised 94.5% of cohorts on time in 2024 and are continuing strong in 2025 with a 93.5% year-to-date randomisation rate. Recognised by Clinical Trials Arena for Marketing and Innovation, our participant recruitment model consistently delivers exceptional results that drive study success.
Our participant experience is second to none, with Net Promoter Scores (NPS) consistently above 70 a powerful indicator of trust and satisfaction. Many of our participants return for future studies, thanks to our dedicated Participant Experience team, personalised communication, and focus on the greater good, comfort and care.
Get in touch today
to discover our Recruitment Excellence Design
Tailored solutions for complex clinical trials, engineered for speed, quality and precision.
More than a place to conduct trials – a partner in your entire clinical research journey. We facilitate every step of your clinical journey, from design to delivery and provide expert guidance on Australia’s innovative clinical trial frameworks, including the fast-track trial pathway and generous R&D incentives.
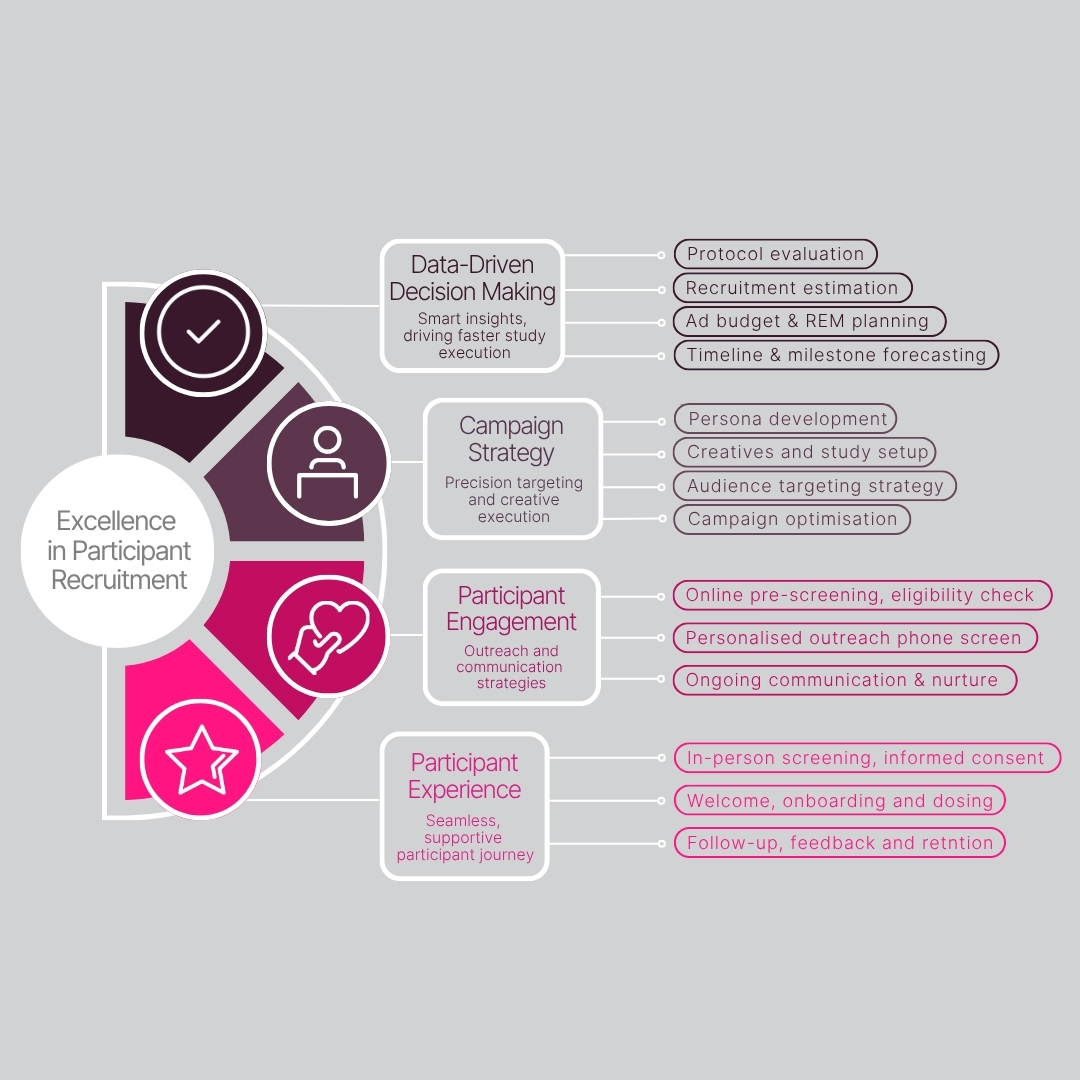
Why Sponsors Trust Linear Clinical Research
At Linear, we don’t just recruit participants, we engineer clinical trial success. Our recruitment and retention strategy is built on experience, innovation, and a deep commitment to delivering reliable, rapid results for biotech and pharmaceutical partners.
Extensive, Curated Participant Database
Our proprietary database includes tens of thousands of pre-screened individuals, ranging from healthy volunteers to niche patient populations, collected through years of dedicated community engagement and digital opt-ins. We can segment by demographics, health conditions, biomarkers, and trial eligibility criteria, enabling precise matching at speed.
Advanced, Data-Driven Digital Enrolment
We utilise a full-funnel digital marketing ecosystem, across Google, Meta, programmatic advertising, and email marketing, backed by A/B testing, lookalike modeling, and behavioural analytics. Our system continuously learns from previous trials to optimise every campaign for higher conversion and lower cost-per-enrolment.
Participant Engagement That Builds Trust
Our Participant Experience Team (PEX) offers personalised onboarding, regular follow-ups, and responsive support throughout the trial journey. This human-centered approach fosters trust, increases retention, and improves compliance, especially in longer or more demanding protocols.
Faster Enrolment Timelines
Thanks to our hybrid model, combining real-world insights, predictive tools, and operational excellence, we consistently deliver first-patient-in (FPI) proven by our year 24 performance of 94.5% and year-to-date 93.5% on time cohorts dosed. Our recruitment timelines are backed by data forecasting and real-time tracking so you always know where things stand.
Driving Retention Through Engagement and Loyalty
Ee ensure participants are well-prepared and committed from the outset. Our tailored retention programs foster strong participant loyalty, keeping them engaged and informed resulting in high protocol adherence, strong retention rates, and fewer dropouts..
Recruitment Excellence Design
We offer sponsors an unmatched level of assurance through our Recruitment Excellence Design an operational commitment that aligns projected recruitment numbers with actual delivery. Our team takes accountability for timelines and proactively adapts to ensure milestones are met.
Global Standards, Local Expertise
Operating out of Australia’s leading medical campuses, we combine globally benchmarked quality with local knowledge of patient communities and healthcare pathways. Our seamless integration of digital, clinical, and engagement capabilities makes us a trusted partner for both early-phase and late-phase studies.
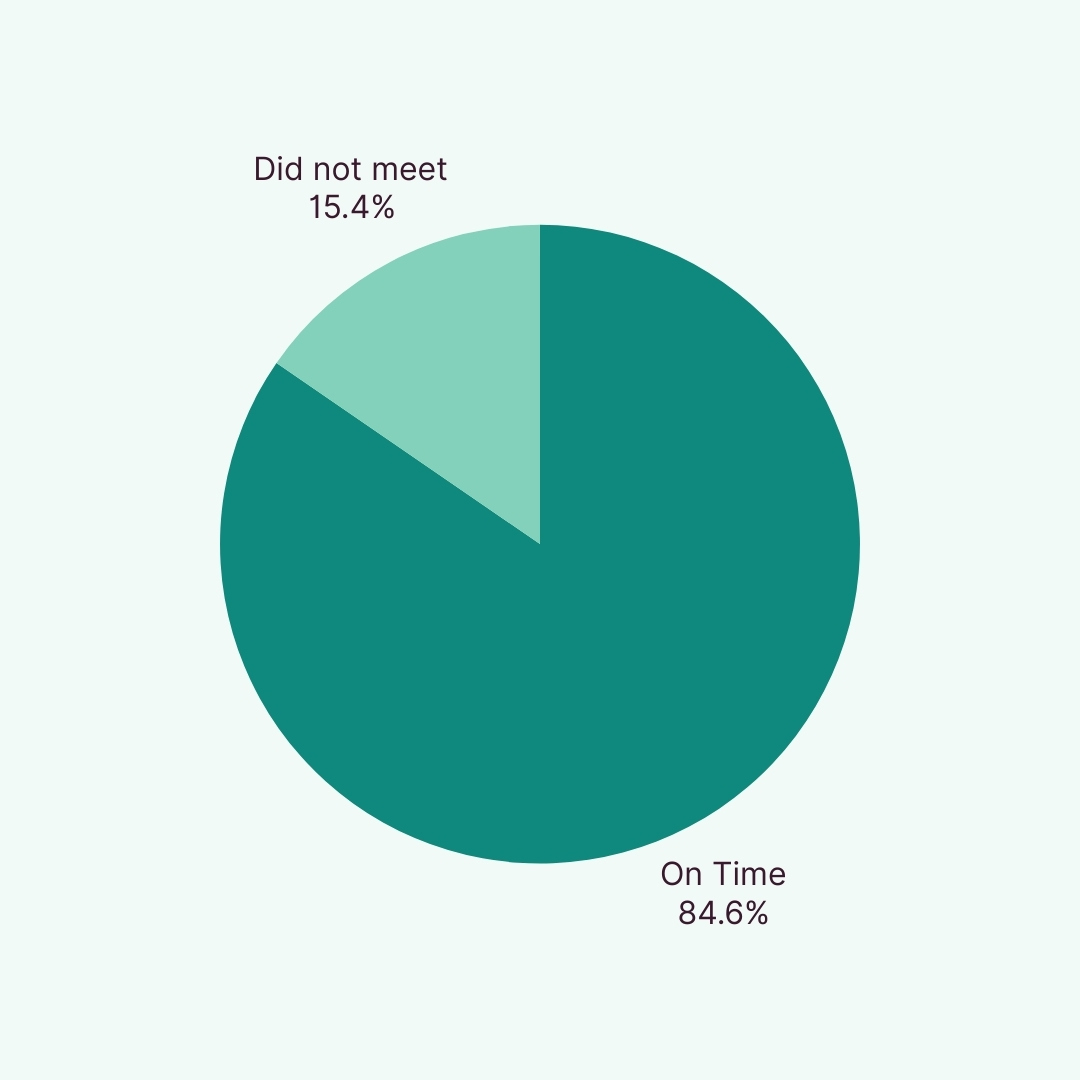
Our Consistent Delivery of On Time Clinical Projects
“Dose on time” is stringent performance measure on how many cohorts were randomised on the scheduled date, in alignment with sponsor-approved timelines.