Delivering Vaccine Trials at Speed and Scale
Delays in recruiting participants, getting regulatory approvals, or preparing study sites can slow down vaccine development in a big way. At Linear, we’ve built our clinical trial services to tackle these challenges head-on.
Since 2010, we’ve conducted over 500 clinical trials, including 18 vaccine studies for international biopharma companies, across a variety of therapeutic areas. With three advanced clinical facilities, we handle trials at every phase, focusing on getting things done quickly while maintaining high standards for quality and data accuracy.
We move studies forward by speeding up ethics and regulatory approvals, targeting recruitment efforts, and managing data effectively. In 2024, 94% of our trial cohorts were dosed on schedule, thanks to our experienced team, thoughtful planning, and modern infrastructure.
By working within Australia’s efficient trial frameworks and tapping into a large, trial-ready population, Linear helps global sponsors move their vaccine programs forward with confidence and precision.
What Sets Our
Vaccine Trials Apart
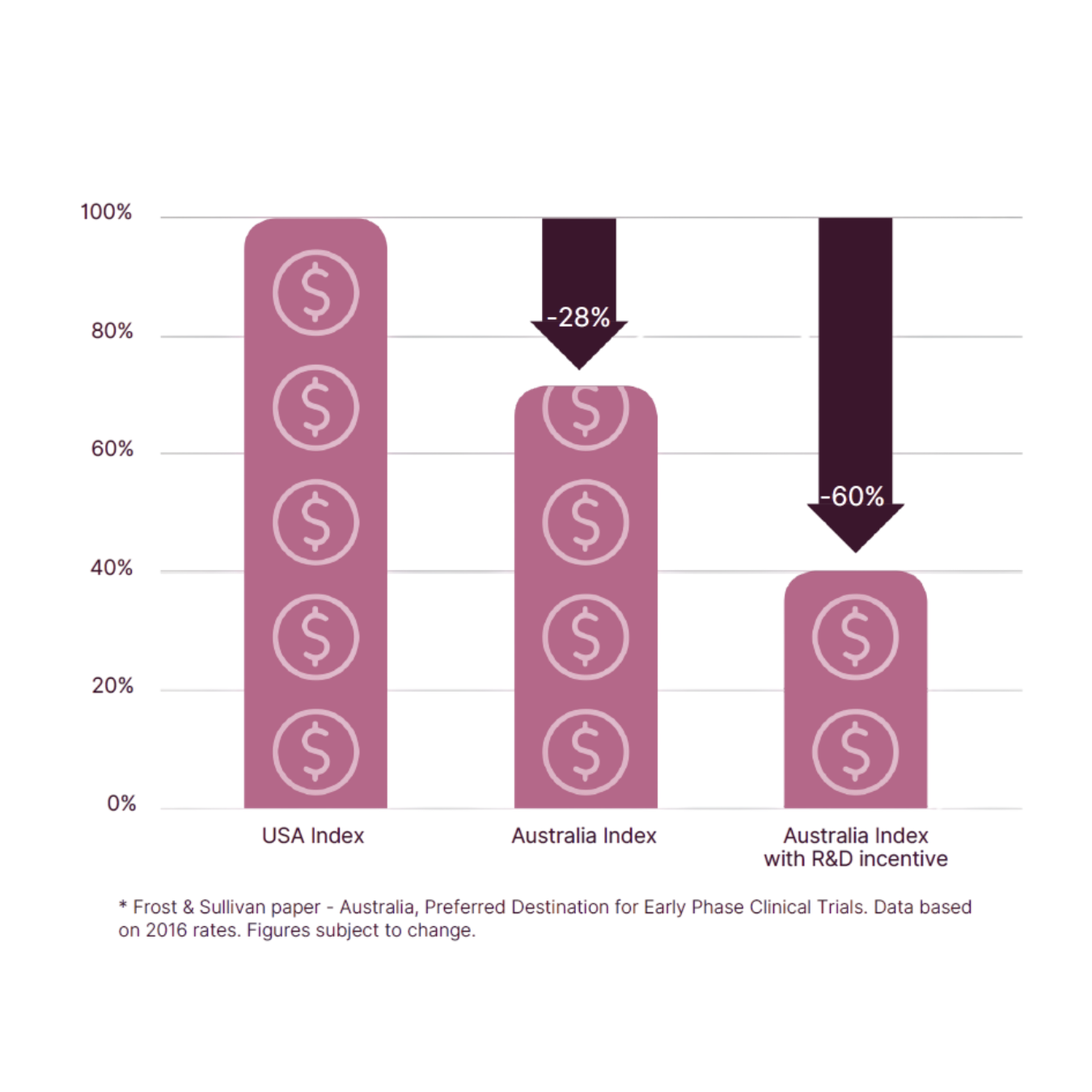
The Australian Advantage - Cost*
Save up to 60% on your clinical trial costs by running your trial in Australiaat Linear The base cost of running a trial in Australia is 28% less, taking intoaccount the value of USD compared to AUD.
Further benefit comes with Australia’s R&D tax incentive. Performing aclinical trial with Linear will allow you to reclaim 43.5% of the whole cost ofyour clinical trial, in cash.
Linear engages a number of strategic partners that can help you leveragethe most out of the incentive, to ensure you have more cash to progressyour product forward.
The Australian Advantage - Speed
Save months on your clinical development timeline by performing your First-in-Human study prior to submitting an IND. The Therapeutic Goods Administration (TGA), uses a simple streamlined regulatory process for clinical trials, allowing you to prepare your IND submission in parallel with the execution of your Phase I at Linear.
The data generated from clinical trials in Australia is wholly accepted by both the EMA and FDA and is considered to be of the same quality as data generated in the EU or US.

Our clinical facilities at a glance
- Nedlands Clinical Trial Centre
- Co-located with a 600-bed tertiary hospital.
- Situated within one of the largest medical precincts in the Southern Hemisphere.
- 24-bed facility purpose-built for Phase I–III clinical trials.
- Specialised in outpatient vaccine studies.
- 24/7 Medical Emergency Team coverage for enhanced safety.
- Joondalup Clinical Trial Centre
- Purpose-built healthy volunteer clinic completed in August 2022.
- Equipped with 24 state-of-the-art beds.
- Integrated telemetry safety monitoring system.
- One of Australia’s newest clinical trial facilities.
- Designed for an innovative, cutting-edge participant experience.
- Advanced Clinical Trials Centre
- Australia’s first accredited private hospital dedicated to clinical trials.
- Accessible to both private and public trial participants.
- Includes six consultation rooms and a processing lab.
- Capacity for 18 participants, including 6 overnight stays.
We were thoroughly impressed with the world class service; the execution of the trial was excellent, the project was completed ahead of schedule.