Applying for a trial
Thinking of contributing to human breakthroughs in WA and have a few questions? Our answers to the most frequently asked questions are set out below.
Have further questions? Please call us on 1300 546 327 or email contactus@linear.org.au we’ll be happy to help!
Question List
If I am healthy, why can I be considered for a trial to treat a particular condition or disease?
Healthy volunteers play an important role in the development of new medications or treatments. Before the treatment can be safely administered to patients with a particular condition or disease, it undergoes a comprehensive testing process that includes “early phase” testing to assess the general safety, and any potential side effects, in people who are healthy so that the precise effects of the treatment can be determined.
How do I volunteer for a clinical trial?
Ready to apply for a trial? Compare our current trials.
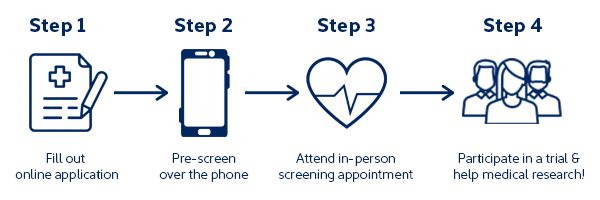
Step 1: Fill Out an Online Application
You can compare our current trials and apply for as many as you like. Pay close attention to the time commitments of the study and the dates you will be required to attend Linear, as well as the key eligibility requirements. The online application will take 5-10 minutes and will ask you about your personal details and some medical information.
Step 2: Pre-screening Over the Phone
The next step will be for one of our friendly recruitment team members to give you a call and confirm the responses you gave in your online application. If you were able to book a screening appointment, we will still need to speak with you over the phone to confirm your appointment.
If you have submitted an application and haven’t heard from us, please see the following FAQ’s:
I applied for clinical trials, but I haven’t heard back?
I applied for a clinical trial, but was told I’m not eligible?
Step 3: Attend an In-Person Screening Appointment
If you are eligible for a screening appointment, our team will arrange a date and time for the appointment, and explain the screening procedures involved – generally, the screening appointment is a comprehensive medical check-up, and your results can be sent to you upon request. The screening team and doctors will determine whether or not you are eligible for the trial based on your results, and contact you to inform you of the outcome. Some trials may require a second appointment, such as an eye test before we can fully determine whether or not you are eligible to participate.
Step 4: Participate in a Trial and Help Medical Research
The last step is to participate in a clinical trial with us! This will involve completing all of the study requirements that the trial you’re participating in consists of. This may include a period of staying in our clinic and attending follow-up appointments, or the trial could be appointment-only study. If you’re curious about what is involved in study participation, jump to our “During a Trial” Questions section. You can also find information in our FAQs about payment for participation.
I applied for a clinical trial, but was told I’m not eligible?
Each trial has its own requirements that determine whether or not an applicant is eligible to participate. Often, trial applicants may not be eligible for a particular trial, but eligible for another, based on the criteria specific to each trial.
General reasons that an applicant may not be eligible for a particular trial they have applied for can include: use of medication that is not allowed for the trial, being outside the age range required for the trial, not meeting the requirements for birth control, or having a particular condition or illness which would make participation in the trial unsafe for the applicant.
Some applicants may not be eligible for any of our Healthy Volunteer trials due to a significant illness or disease but may be eligible for a patient trial targeting their condition.
Please note that applicants with a recent history of recreational drug use, drug abuse or alcoholism, or recent major surgery, will not be eligible for any of our trials until a certain “washout period” has passed.
If you applied for a trial and are unsure of why you were not eligible, you are welcome to contact our Recruitment team on 1300 546 327 or get in touch via Contact Us.
Why is birth control required for clinical trials?
Birth control is required to participate in a clinical trial, for the duration of the trial and often for some time after the trial is complete, to prevent any possibility of a pregnancy occurring while a participant is involved in a trial. This is because it is not known how a new study drug may affect an unborn child.
If you would like more information surrounding birth control eligibility requirements, please call our friendly Participants Experience Team on 1300 546 327 or visit Contact Us.
What happens if I don’t want to participate in a trial anymore?
All participants have the right to withdraw from a trial at any point in the study, for any reason. You do not have to give a reason for ending your participation in a trial, If you choose to withdraw your consent to participate in the study, then you will receive a partial payment based on your contribution up to the point where you withdraw your consent as outlined in the Participant Infomation Consent Form. Additionally, if you withdraw from a trial for a particular reason, you may still be able to apply for a different trial if you would like to, depending on the circumstances.
If you are a patient who was referred to participate in a clinical trial by your doctor, withdrawing from the trial will not affect your ability to access your usual care.
If you would like more information about withdrawing from a study, please call our friendly Recruitment Team on 1300 546 327 or get in touch via Contact Us.
Can I participate in more than one trial?
Participants may only participate in one trial at a time, but many choose to return to Linear once they have completed a trial. If you would like to participate in multiple trials or have recently completed a trial where you received an investigational product (either with us or another organisation), there is a mandatory “wash-out” period before you can participate in another one. This is generally 30 to 90 days. If you would like to participate in another trial but are unsure if you may be eligible, our Recruitment team can advise you on 1300 546 327 You can also get in touch with us via Contact Us.
I applied for a clinical trial, but I haven’t heard back?
Each trial has its own requirements that determine whether or not an applicant is eligible to participate. Often, trial applicants may not be eligible for a particular trial, but eligible for another, based on the criteria specific to each trial.
General reasons that an applicant may not be eligible for a particular trial they have applied for can include: use of medication that is not allowed for the trial, being outside the age range required for the trial, not meeting the requirements for birth control, or having a particular condition or illness which would make participation in the trial unsafe for the applicant.
Some applicants may not be eligible for any of our Healthy Volunteer trials due to a significant illness or disease, but may be eligible for a patient trial targeting their condition.
Please note that applicants with a recent history of recreational drug use, drug abuse or alcoholism, or recent major surgery, will not be eligible for any of our trials until a certain “washout period” has passed.
If you applied for a trial and are unsure of why you were not eligible, you are welcome to contact our Recruitment team on 1300 546 327 or get in touch via Contact Us. You can also find another trial here that you may be eligible to participate in.
Can I participate in a trial at Linear if I don’t live in Perth?
All of our trials are based in Perth, and require a screening appointment prior to determining whether or not you can participate in the trial. This means that you would need to be in Perth not only for the trial period itself, but for screening appointments prior to the trial beginning. Additionally, due to the nature of clinical trials, dates for each trial can change – while we do our best to accommodate your preferred dates, it is not always possible.
For this reason, it is not possible for participants who are based overseas or interstate to participate unless in Perth for an extended period of time.
Participants who live regionally in Western Australia and are confident they can commit to the requirements of the trial may apply.
If you are unsure of whether or not you may be able to participate in a trial, our Recruitment team can advise you on 1300 546 327 or get in touch with us via Contact Us.
Why are you only recruiting males/non-smokers/etc?
Each clinical trial has different eligibility requirements depending on a range of factors including: previous trials carried out and their data, sponsor requirements, participant safety and so on. These criteria are put in place to make sure the data collected in a study is relevant and builds on previous research, and not for the purpose of restricting certain demographics from participating.
You can compare our current trials and apply for any that suit you, here or you can register to our mailing list and be the first to know about new trials, here.
I signed up, what happens next?
Once you have filled out one or more of our online applications, the next step will be for one of our friendly recruitment team members to give you a call and confirm the responses you gave in your application. As our trials are very popular and we regularly receive more applications than we need, our Recruitment Team is only able to contact eligible or likely-eligible applications.
If you are found to be ineligible, we will not call you to confirm this. If you believe you have been found ineligible in error, please contact our Recruitment Team directly to discuss your eligibility further.
If you were found to be eligible, please allow at least one week for us to get in touch with you. If you submitted an application over a week ago and haven’t heard from us, please see the following FAQ’s:
I applied for clinical trials, but I haven’t heard back?
I applied for a clinical trial, but was told I’m not eligible?
You can also follow your application up with us by getting in touch with us yourself and we may be able to progress your application.
